本帖最后由 hzangs 于 2015-12-14 14:27 编辑
版权归属外泌体之家,未经书面许可,禁止转载。
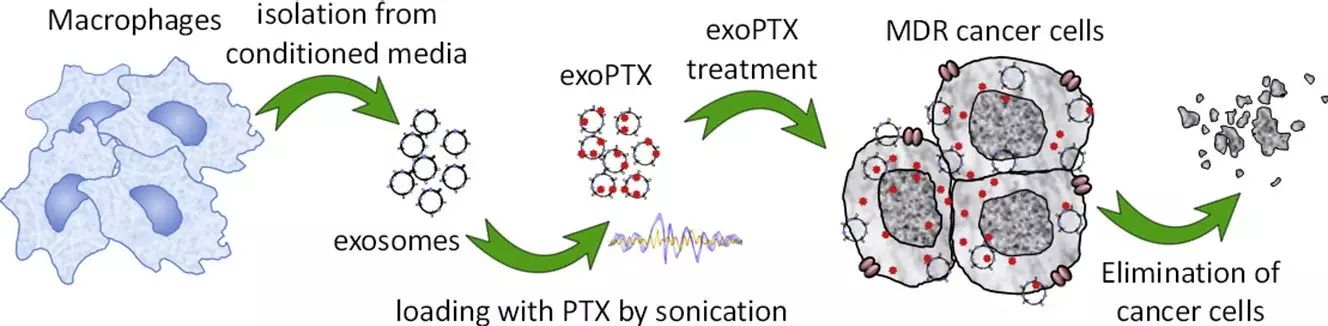
近来,外泌体(Exosomes)被很多人作为一种“天然的纳米粒子”来进行药物传送。来自北卡罗来纳大学教堂山分校的研究者提出了基于外泌体作为药物运载工具的可能性,从而提高化疗药物紫杉醇对耐药肿瘤细胞的治疗效果。因此,研究者们利用巨噬细胞分泌的外泌体,寻找并比较了各种外泌体运载紫杉醇(exoPTX)的方法,进而对这个外泌体-紫杉醇复合物的大小、稳定性、药物释放以及抗肿瘤效果进行了描述。研究者们还发现,对外泌体的膜结构进行超声处理会提高外泌体的最大药物承载量并有持续的药物释放。重要的是,外泌体包被紫杉醇会对耐药细胞系MDCKMDR1 (Pgp+) 增加超过50倍的细胞毒性。然后,该研究在小鼠Lewis肺癌转移模型中发现该运载外泌体与肿瘤细胞有几乎完全的共定位,并在小鼠模型中有潜在的抗肿瘤效应。因此,研究者得出结论,利用外泌体运载紫杉醇(exoPTX)的方法,可能对各种耐药肿瘤细胞有着很好的潜在化疗效果。
Kim MS et al. (2015) Development of Exosome-encapsulated Paclitaxel to Overcome MDR in Cancer cells. Nanomedicine [Epub ahead of print].
Exosomes have recently come into focus as “natural nanoparticles” for use as drug delivery vehicles. Researchers at the University of North Carolina at Chapel Hill set out to assess the feasibility of an exosome-based drug delivery platform for a potent chemotherapeutic agent, paclitaxel (PTX), to treat MDR cancer. Herein, they developed and compared different methods of loading exosomes released by macrophages with PTX (exoPTX), and characterized their size, stability, drug release, and in vitro antitumor efficacy. Reformation of the exosomal membrane upon sonication resulted in high loading efficiency and sustained drug release. Importantly, incorporation of PTX into exosomes increased cytotoxicity more than 50 times in drug resistant MDCKMDR1 (Pgp +) cells. Next, the studies demonstrated a nearly complete co-localization of airway-delivered exosomes with cancer cells in a model of murine Lewis Lung Carcinoma pulmonary metastases, and a potent anticancer effect in this mouse model. The researchers conclude that exoPTX holds significant potential for the delivery of various chemotherapeutics to treat drug resistant cancers.
---------------分割线--------------- 文章原文在3楼
|  /1
/1 
 |Archiver|手机版|外泌体之家 | exosomes & microvesicles
|Archiver|手机版|外泌体之家 | exosomes & microvesicles
